Bloodworks Research Institute
Bloodworks Intravital Microscopy Imaging Core

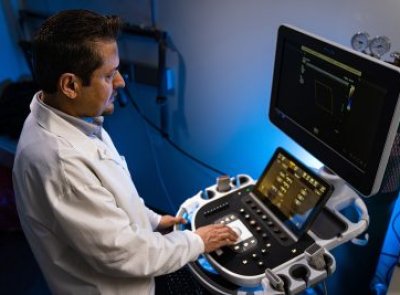
The Imaging Core at Bloodworks Research Institute was established to promote interdisciplinary collaborations and translational research using live research subjects. The Imaging Core has three major equipment for in vivo imaging studies: 1) an upright confocal intravital microscope, 2) an inverted intravital microscope, and 3) contrast-enhanced ultrasound molecular imaging systems (see details below). These instruments enable investigators to monitor blood circulation, blood cell-vessel wall interactions in both large and small vessel systems (arterial, venous, and microcirculation), and microvascular blood perfusion in various organs. The real-time visualization of cellular processes under physiological and pathological conditions, such as vascular injury and thrombo-inflammatory disease, will provide key model data for multiple research areas, including hematology, immunology, cancer, and drug testing.
Expertise
The Imaging Core staff have extensive expertise in performing basic and translational research using model systems, which includes experimental design, real-time in vivo imaging, image processing, and data analysis. We have established various mouse models of thrombosis, hemostasis, and inflammation (see details below).
Instruments


Upright 5-line Zeiss Axio Examiner high-speed confocal intravital microscope
This system is equipped with a heated stage, a solid laser launch system at 405nm, 488nm, 561nm, and 638nm (LaserStack; Intelligent Imaging Innovations), CSU-W1 Spinning Disk confocal unit, a high-speed CMOS camera, and a microperfusion pump. This intravital microscopy system is custom designed for direct observation of blood cells: platelets, leukocytes, RBCs, and plasma protein-vascular wall endothelial interactions in a variety of vascular beds and organs. Assays available include:
- FeCl3-induced carotid artery thrombosis model in model systems: thrombosis model in a large vessel to monitor platelet-vessel wall interaction, dynamics of thrombus growth, stability, and vessel occlusion. (Video)
- Laser-induced cremaster arteriole thrombosis model: Quantitative analysis of platelet accumulation, fibrin formation, and other blood proteins within a growing thrombus in the microvasculature. (Video)
- Leukocyte rolling, adhesion, transmigration, platelet-neutrophil aggregation in cremaster venules; Leukocyte-platelet aggregates in venous and arteriole clots.
- Laser-ablation bleeding model in cremaster arteriole: assess clot formation and blood loss (Video)
- Electrolytic Inferior Vena Cava thrombosis model (EIM): Deep vein thrombosis model
- Laser-ablation saphenous hemostasis model: assess the dynamics of platelet-fibrin hemostatic clot formation (Video)
- Monitoring cerebral and lung microvascular blood circulation
- VWF-induced cerebral microvascular thrombi
- VWF-mediated microvascular thrombosis model: This model is used to study the pathobiology of microangiopathy in the brain and other organs and test novel therapeutics. (Video)
Inverted Zeiss Axio Observer Z1 Marianas Fluorescence Microscope
This system is equipped with a custom heating stage, high-speed CMOS camera, 4-line fluorescence, and DIC uniquely designed for conducting in vivo hemostasis and thrombosis assays including:
- Mesenteric arteriole thrombosis model: Vascular injury-induced thrombosis model in microvessel to monitor platelet vessel wall interaction, dynamics of thrombus growth, stability, and vessel occlusion
- Leukocyte rolling, adhesion, transmigration, and platelet-neutrophil aggregation in mesenteric venules in response to inflammatory stimuli
- Monitoring microcirculation in the liver and spleen
- Ionophore-provoked mesenteric ULVWF-mediated microvascular thrombosis (Video)
- Microscopic real-time imaging of platelet adhesion and spreading ex vivo (human and mouse and other species)
- Microscopic real-time imaging of platelet adhesion and thrombosis in a perfusion chamber under shear (human and mouse whole blood)
Contrast-enhanced ultrasound molecular imaging systems
EPIQ Elite Diagnostic Ultrasound System (Philips Healthcare) is specifically designed to examine vital organs in model systems. Using non-target microbubbles this system can access microvascular blood perfusion for a global assessment of organ function. Using targeted microbubbles allows the system to image the molecular components of thrombosis that cause impaired microcirculation and potentially can be used to deliver novel therapeutics. Assays available include:
- Ultrasound imaging of the heart, liver, spleen, and other organs
- Contrast-enhanced microvascular circulation of the heart, liver, and kidney
- Contrast-enhanced ultrasound imaging of skeletal muscle microvascular perfusion
- Molecular imaging of cardiac microvascular thrombosis using VWF-platelet targeted microbubbles
Olympus dissecting microscope: The system has a heated surgical platform for microvascular surgery and a digital display system for training.
Selected images from in vivo models showing arterial thrombosis, bleeding, hemostatic clot formation, and microvascular thrombosis formed in the brain and mesenteric vessels.
Image 1: Laser-induced cremaster arteriole thrombosis model
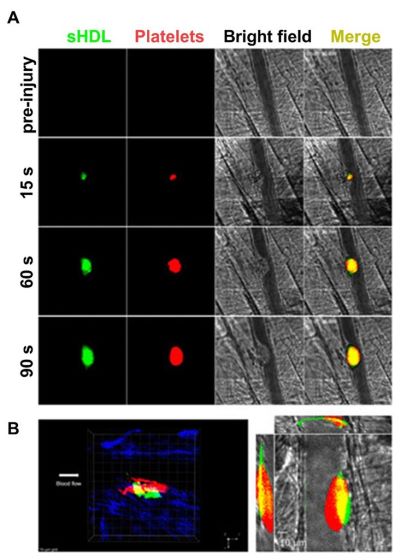
Image 2: FeCl3-induced carotid artery thrombosis model
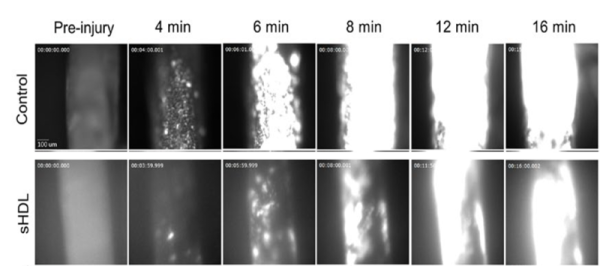
Image 3: Laser-Ablation Cremaster Arteriole Rupture Hemostasis Model
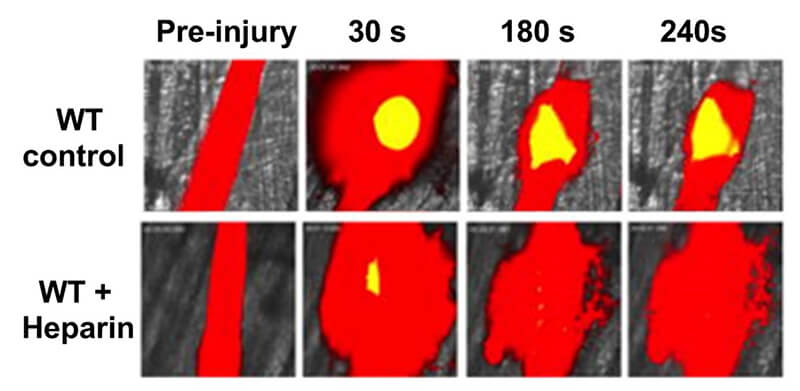
Image 4: Laser-Ablation Cremaster Arteriole Rupture Hemostasis Model
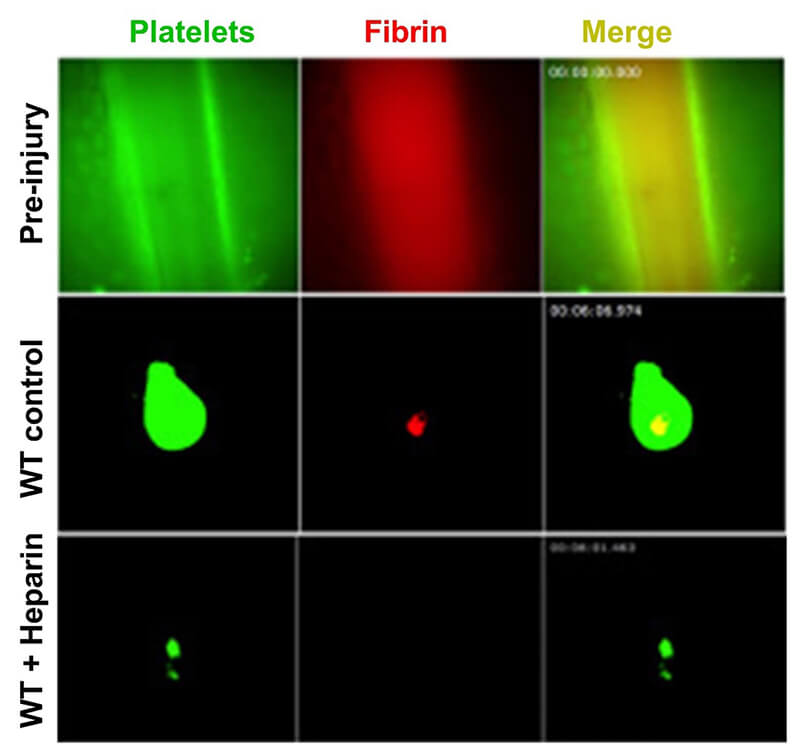
Image 5: VWF-mediated microvascular thrombosis model
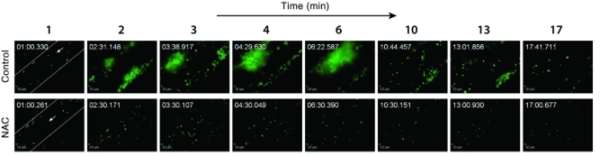

Raymond Adili, M.D.
Associate Member
Director of Intravital Microscopy Imaging Core
Bloodworks Northwest
Research Institute
Bloodworks Northwest
Research Institute
1551 Eastlake Avenue E, Suite 100
Seattle, WA 98104
Email: [email protected]
Phone: (206) 689-6346
FAX: 206-587-6056
Tena Petersen
Senior Administrative Coordinator
Email: [email protected]
Phone: (206) 568-2246
Fax: (206) 587-6056
Accessing Services
Imaging Core is open to all investigators within and outside Bloodworks, including industry partners. To discuss the details of your research project and evaluate its feasibility, please contact Dr. Reheman Adili at [email protected], office phone: (206) 689-6346, or Imaging Core phone: 206-568-2233.


